Description
Comprehensive PubChem-Based Research Overview
Molecular Identity & Classification
5-Amino-1MQ, formally known as 5-amino-1-methylquinolinium, is cataloged in PubChem under CID 1373493 with the molecular formula C10H11N2+ [1]. It belongs to the quinolinium class of heteroaromatic compounds and is structurally characterized by a quaternary nitrogen substituent at the 1-position of quinoline. This molecular identity provides the basis for its role as a selective NNMT inhibitor in chemical biology research.
Mechanism of Action: NNMT Inhibition
NNMT catalyzes the transfer of a methyl group from S-adenosylmethionine (SAM) to nicotinamide, thereby modulating NAD+ metabolism and cellular methyl donor balance. By blocking NNMT, 5-Amino-1MQ alters downstream nicotinamide and NAD+ fluxes, enabling researchers to dissect pathways of methylation control, redox balance, and metabolic regulation [1].
Research Applications in Cellular Metabolism
- NAD+ and Energy Studies: NNMT activity has been implicated in regulating intracellular NAD+ pools. Using 5-Amino-1MQ in cultured cells allows quantification of NAD+-linked enzymes and mitochondrial function under inhibited NNMT states [1].
- Methyl Donor Availability: Inhibition of NNMT shifts the use of SAM, permitting exploration of epigenetic methylation patterns and histone/DNA methylation status in cell-based assays [1].
- Metabolic Modeling: Experimental use of 5-Amino-1MQ in metabolic flux assays supports studies of obesity, insulin resistance, and altered energy expenditure, though such contexts remain strictly preclinical and mechanistic [1].
Chemical Biology & Assay Development
5-Amino-1MQ’s PubChem entry provides registry identifiers, structure files, and depositor data, ensuring traceability for:
- Screening libraries focused on NNMT pathway modulators.
- Structure–activity relationship (SAR) studies comparing quinolinium analogs.
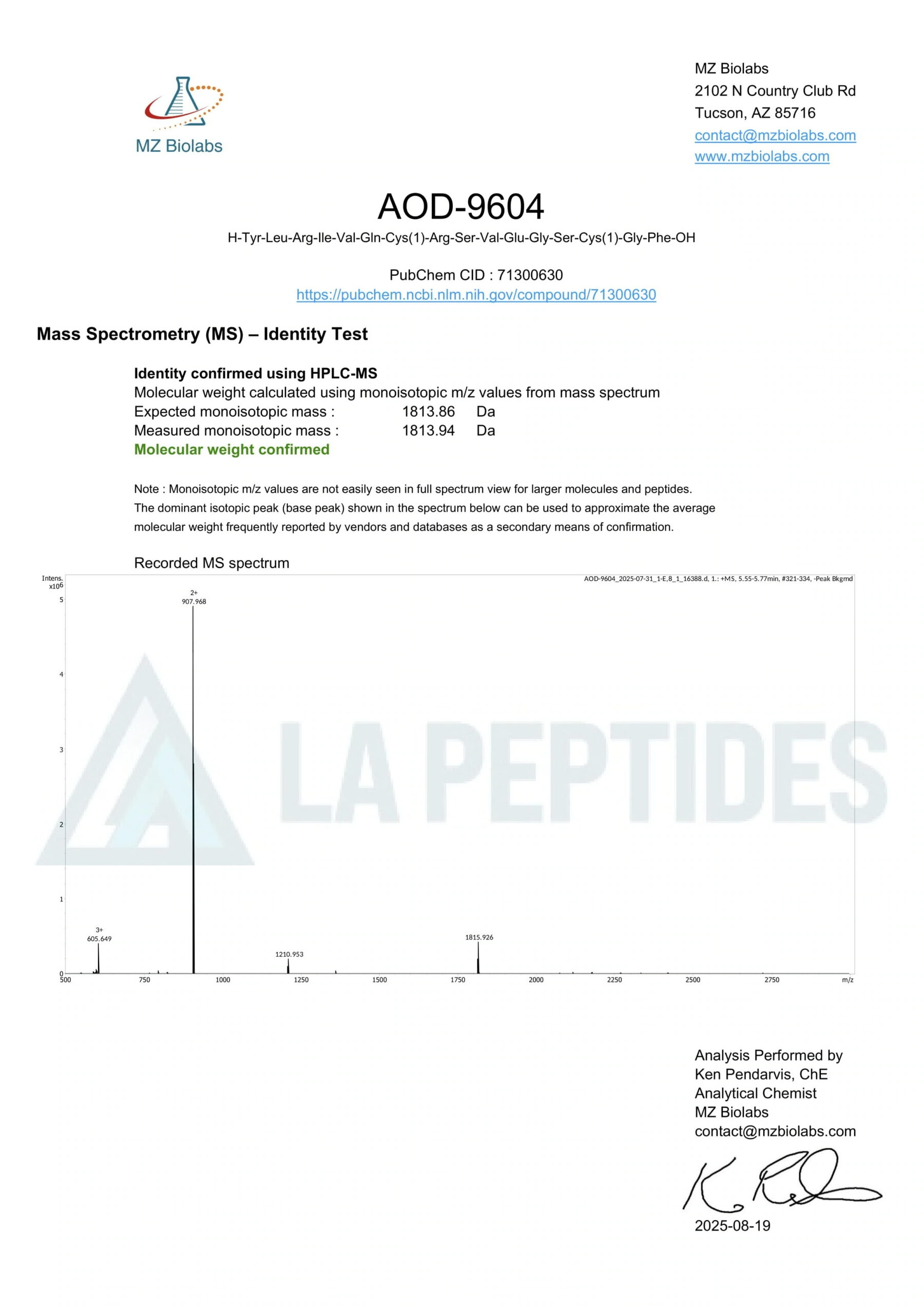
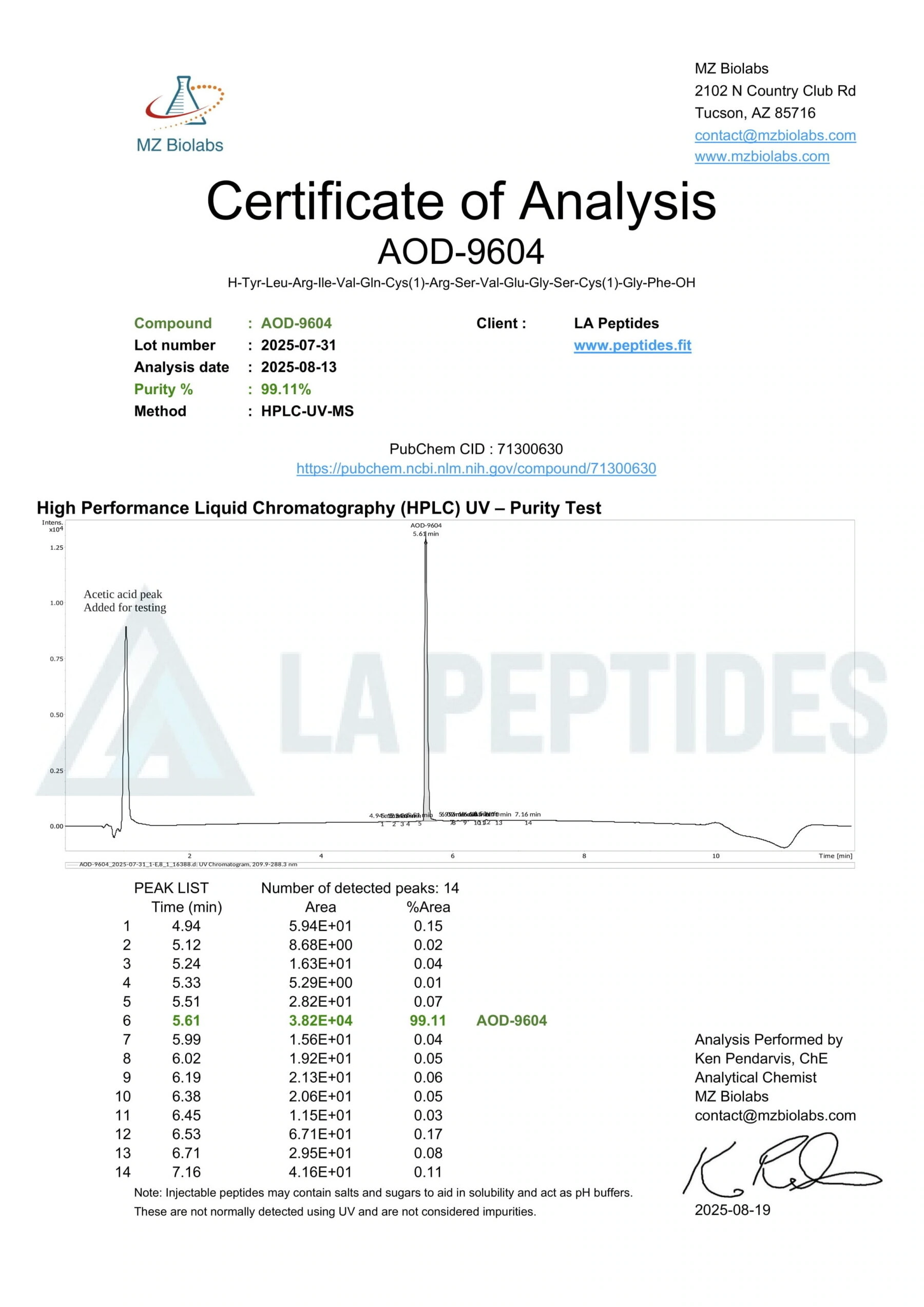
- Analytical QC via NMR, MS, and chromatographic purity confirmation.
Physicochemical Characteristics
According to PubChem, 5-Amino-1MQ has a monoisotopic mass of ~159.09 g/mol, is classified as a quaternary ammonium compound, and exhibits physicochemical features consistent with cationic solubility profiles. These characteristics guide solubility and formulation considerations in in vitro experiments [1].












There are no reviews yet.